- Number Of Electrons Gained Or Lost In Helium
- Number Of Electrons In Helium Element
- Element With 4 Electrons
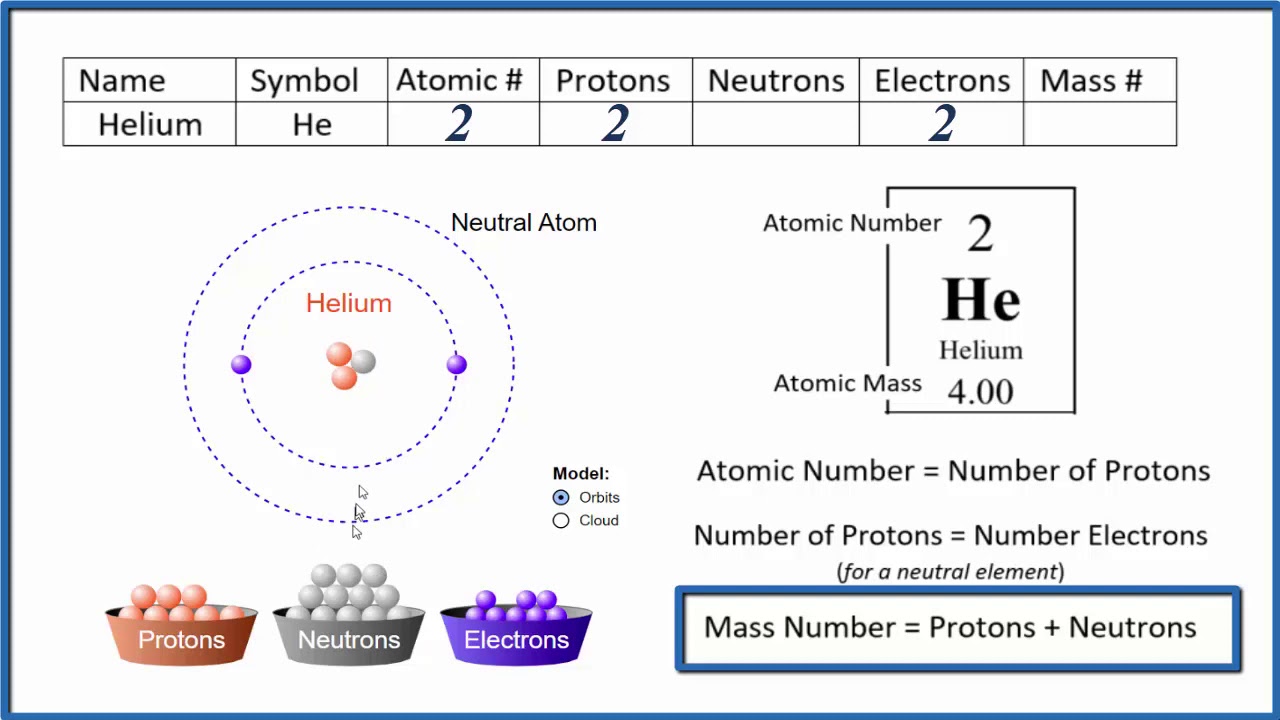
Vray 3ds max 2016 crack. Helium is a chemical element with the atomic number 2, meaning that a neutral helium atom has two protons and two electrons. The most important chemical properties of helium include its atomic mass, state of matter, boiling and melting points, and density. The element has an atomic mass of 4.0026 grams per mole and is a gas at almost all temperatures and pressure conditions. The density of helium is 0.1786 grams per liter at 32°F (0.0°C) and 101.325 kilopascals (kPa).
On the contrary, in the laser field any number of photons can be absorbed, leading to an almost continuous energy distribution of the electrons (right-hand panel in Fig. Structure of individual ATI (above threshold ionization) peaks spaced by the photon energy (1.5 eV) is not seen here. Helium is composed of two electrons in atomic orbitals surrounding a nucleus containing two protons and (usually) two neutrons. As in Newtonian mechanics, no system that consists of more than two particles can be solved with an exact analytical mathematical approach (see 3-body problem) and helium is no exception.

Liquid and solid helium can exist only in extremely low-temperature high-pressure conditions. One of the unusual properties of helium is that it cannot exist as a solid or liquid at normal pressures, even at extremely low temperatures. At a pressure of roughly 360 pounds per square inch (2.5 megapascals), the transition between liquid and solid, or the melting point, is -458°F (0.95 Kelvin). The boiling point is -452°F (4.22 Kelvin).
Number Of Electrons Gained Or Lost In Helium
Some of the properties of helium make it an interesting and common subject of study in quantum mechanics. It is, because of its low atomic number, the second simplest atom after hydrogen. Mathematical procedures can be used to analyze the behaviors of the subatomic particles — protons, electrons, and neutrons — within the helium atom. Such methods cannot, however, determine the behavior of these particles with absolute certainty. Atoms with larger atomic numbers, which have more subatomic particles, tend to be harder to work with in terms of quantum mechanical analysis.
Number Of Electrons In Helium Element
Helium is the least reactive of all of the elements. The nonreactive properties of helium arise from the fact that it is the lightest of the generally-nonreactive noble gases. A noble gas has a 'full' electron shell, meaning that it cannot easily give or receive electrons in a chemical reaction. Electron exchange or sharing is the basis for most chemical reactions, so the noble gases tend to participate in few chemical reactions. Additionally, helium has only two electrons that could participate in a reaction at all, while all of the other noble gases — and indeed, all elements aside from hydrogen — have more.
Element With 4 Electrons
There are many different uses for helium that arise from the chemical properties of helium — particularly its light weight, temperature and pressure qualities, and its low reactivity. Helium is, for instance, considerably lighter than air, so it is often used to fill balloons so they can float and airships such as blimps so they can fly. Liquid helium, which can only exist at extreme pressures and at very low temperatures, is used as a coolant for superconductors, which only take on their extremely conductive properties at very low temperatures.
