Contrary to the beliefs of generations of chemistry students, Avogadro’s number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856). Avogadro was a lawyer who became interested in mathematics and physics, and in 1820 he became the first professor of physics in Italy. Avogadro is most famous for his hypothesis that equal volumes of different gases at the same temperature and pressure contain the same number of particles.
The Avogadro constant is named after the Italian scientist Amedeo Avogadro (1776–1856), who, in 1811, first proposed that the volume of a gas (at a given pressure and temperature) is proportional to the number of atoms or molecules regardless of the nature of the gas. Avogadro’s number is the quantity that dictates the size of atoms and molecules. Thus, the existence of atoms was established when Perrin used Eq.(1) to flnd that NA is in fact a very large number. In this experiment, we follow Perrin and examine the validity of Eq.(1) using 100 nm gold nanoparticles and 1 „m polystyrene spheres in a total of. Acrobat x for mac free. Lightroom enfuse plugin free download. Need for speed hot pursuit reloaded activation key.
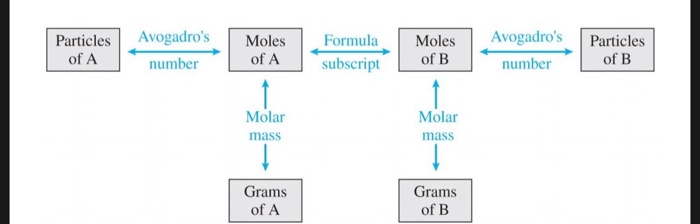
Using Avogadro's Number To Find Grams

The first person to estimate the actual number of particles in a given amount of a substance was Josef Loschmidt, an Austrian high school teacher who later became a professor at the University of Vienna. In 1865 Loschmidt used kinetic molecular theory to estimate the number of particles in one cubic centimeter of gas at standard conditions. This quantity is now known as the Loschmidt constant, and the accepted value of this constant is 2.6867773 x 1025 m-3.
The term “Avogadro’s number” was first used by French physicist Jean Baptiste Perrin. In 1909 Perrin reported an estimate of Avogadro’s number based on his work on Brownian motion—the random movement of microscopic particles suspended in a liquid or gas. In the years since then, a variety of techniques have been used to estimate the magnitude of this fundamental constant.
Avogadro's Number Practice Problems
Accurate determinations of Avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales using the same unit of measurement. This became possible for the first time when American physicist Robert Millikan measured the charge on an electron. The charge on a mole of electrons had been known for some time and is the constant called the Faraday. The best estimate of the value of a Faraday, according to the National Institute of Standards and Technology (NIST), is 96,485.3383 coulombs per mole of electrons. The best estimate of the charge on an electron based on modern experiments is 1.60217653 x 10-19 coulombs per electron. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadro’s number of 6.02214154 x 1023 particles per mole.
Using Avogadro's Number Aleks
Another approach to determining Avogadro’s number starts with careful measurements of the density of an ultrapure sample of a material on the macroscopic scale. The density of this material on the atomic scale is then measured by using x-ray diffraction techniques to determine the number of atoms per unit cell in the crystal and the distance between the equivalent points that define the unit cell (see Physical Review Letters, 1974, 33, 464).

